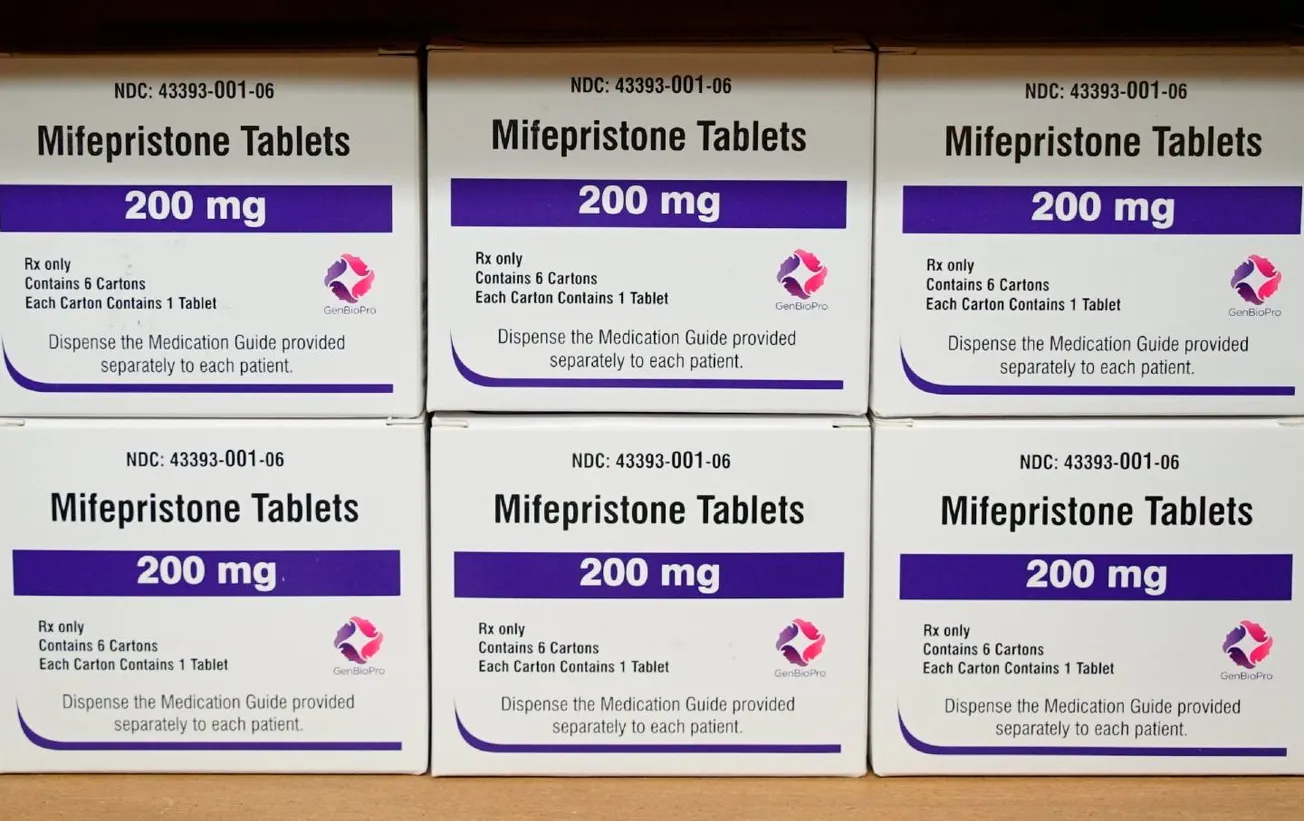
On Friday, April 21, the Supreme Court ruled that the legal abortion drug mifepristone will remain on the market for the time being, temporarily resolving dueling federal district court rulings from Texas and Washington state.
The drug has been in the public eye for months, following increased demand for the pill after the overturning of Roe v. Wade by the U.S. Supreme Court last summer. While 15 states have now banned most abortions, residents in some of them can obtain mail-order abortion medications, partially due to in-person restrictions stemming from the COVID-19 pandemic.
Now, a 7-2 decision from the same high court—with Justices Samuel Alito and Clarence Thomas dissenting—prevents implementation of a Texas’ lower court ruling in Alliance for Hippocratic Medicine v. FDA that said mifepristone was not properly approved by the Food and Drug Administration (FDA). The case, brought by conservative medical activist groups (including at least one Catholic-influenced org), must now go through the appeals process.
On April 10, the Justice Department had filed an emergency motion for a stay of Texas Northern District Judge Matthew Kacsmaryk’s restrictive ruling in Alliance v. FDA. A panel of judges from the U.S. Court of Appeals for the Fifth Circuit then temporarily blocked part of Kacsmaryk’s decision but allowed his restrictions on the ability to order mifepristone by mail or use the drug up to ten weeks into a pregnancy. These prompted an appeal to the Supreme Court from the DOJ and Danco Laboratories, the U.S. manufacturer of mifepristone.
The high court’s decision on the matter had been expected on April 19, before a delay from Alito for an additional two days. Accompanying Friday’s order, his dissent featured an about-face on whether the high court should grant quick stays on high-profile cases.
“If they were warranted in the cases in which they were made, they are emphatically true here,” he wrote, arguing that the Biden-led FDA is attempting to game the legal system to achieve “a desired policy.”
“[A stay] should not be given if the moving party has not acted equitably, and that is the situation here.”
Alito's dissent does not even attempt to defend the decisions by Kacsmaryk or the 5th Circuit. Instead, he condemns the court for using the shadow docket after so many justices criticized it. He specifically calls out Kagan, Sotomayor, and Barrett. https://t.co/Fmn64gkbOS pic.twitter.com/Ek7keV1J69
— Mark Joseph Stern (@mjs_DC) April 21, 2023
At the heart of the case is Danco, which initially intervened in the Alliance v. FDA case in early February. It is only the latest company to market mifepristone, following attempts by various companies between 1988 and 1999 (including a German multinational, Hoechst AG, which for years curtailed distribution expansion on the orders of its Catholic chairman Wolfgang Hilger). In 2000, Danco received rights to U.S. sales of the drug and gained approval from the FDA to market it as Mifeprex.
The same day as Kacsmaryk’s original ruling restricting the drug, a district judge in Washington state, Thomas O. Rice, ruled in a separate lawsuit that mifepristone should remain available without restriction in 16 states and the District of Columbia. That case, filed in January against the FDA by more than a dozen Democratic state attorneys general, challenged pre-Alliance FDA regulations that allegedly made the drug too difficult to obtain.
Reactions to Friday's Supreme Court decision have been mixed, with some hailing it as a triumph of reproductive rights, and others calling it an uncharacteristically hands-off approach from an otherwise hardline conservative bench.
Maryland Attorney General Anthony Brown, a Black Catholic who joined the nation’s Democratic state attorneys general with a February letter supporting pharmacies that provide abortion pills, hailed the April 21 decision as “a victory.”
“The Supreme Court got it right with its decision to protect this critical access while the legal battles play out over the coming weeks. I will remain at the front of those legal battles,” he wrote on social media.
The same day, Rep. Adriano Espaillat of New York—the U.S. Congress’ lone Black Catholic—took to Twitter to celebrate that mifepristone is still “safe, legal, and accessible.”
“We will never stop fighting to protect reproductive choice.”
Bishop Michael Burbidge of Arlington, chair of the U.S. Conference of Catholic Bishops’ pro-life committee—which focuses mostly on abortion—expressed disappointment with the decision.
“The FDA acted unlawfully when it first approved, and later relaxed safety requirements for prescribing and dispensing the drug. It is our hope and prayer that the Court will eventually overturn the FDA's improper actions,” he said.
“Abortion is never the answer for a difficult or unintended pregnancy, as it always ends one life and risks another. Meaningful compassion for both mothers and children is needed.”
Following the Supreme Court decision in Alliance, the DOJ appeal of the Texas ruling now returns to the U.S. Court of Appeals for the Fifth Circuit, which is expected to begin hearing arguments on Wednesday, May 17.
Nate Tinner-Williams is co-founder and editor of Black Catholic Messenger and a seminarian with the Josephites.